What is a tetrahedral coordination geometry that has salen ligand in it and is paramagnetic.
Crystal field theory (CFT) is a simple model used to explain the bonding and account for the characteristic optical and magnetic properties of coordination complexes. In a few sentences (no more than 150 words) and using any relevant illustration explains the tenets of CFT. (6 marks) b) Choose two complexes, one of an octahedral coordination geometry and the other of tetrahedral coordination geometry that are diamagnetic and paramagnetic respectively, that result from coordination between any two of the metal ions Cr2+, Mn²+, Fe²+, Co2+, Ni2+ or Fe+ and any two of the ligands, I, II, and III. For these two complexes apply CFT to evaluate the following: include in your discussion completely labelled crystal field splitting diagrams and calculations 1. their coordination geometry (draw structures), (4 marks) 2. the colour observed for the octahedral complex, (3 marks) 3. the nature of the magnetism observed, and (6 marks) (determine) which one is more stable. (6 marks) NB. Units for D, or D. should be in kJ mol' (use Avogadro's number to convert) and note units for Imax.
N II NE N *O III Fe Zhou, X., et al. Nat Commun 10, 1488 (201 Ni2+ +270 Co2+ +250 Fe2+ pnergy (P) for select first row transition metal ions. TRN TO THE

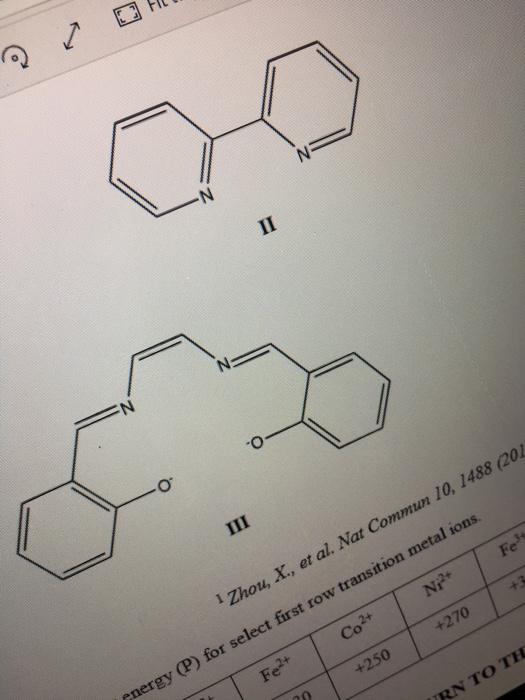
The ligands that I use too is a salen ligand and a 2,2' bi pridine. the ligands are as above

Login to view answer.