Want to check the last two on the first page to see if they're correct but hav...
consider f(x)=z-2z^2. identify the real and imaginary parts of f
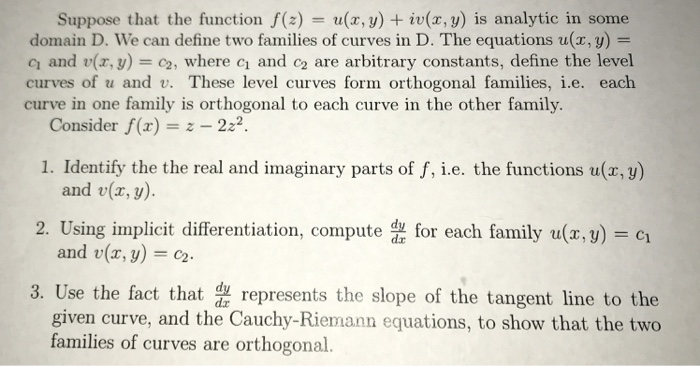
Suppose that the function f(z) = u(x,y) + iu(x,y) is analytic in some domain D. we can define two families of curves in D. The equations u(x, y) = q and v(x,y) = c2, where c1 and c2 are arbitrary constants, define the level curves of u and v. These level curves form orthogonal families, i.e. each curve in one family is orthogonal to each curve in the other family. Consider f(z) 222 1. Identify the the real and imaginary parts of f, i.e. the functions u(r, y) and v(x, y). and u(x, y) = c2. given curve, and the Cauchy-Riemann equations, to show that the two 2. Using implicit differentiation, como family u(.) 3. Use the fact that represents the slope of the tangent line to the families of curves are orthogonal.


Teport) You must be able to perform these calculations with given data on your Volume of KMnO Subtract the initial buret volume from the final buret volume. concept review quiz. 17.45hL -21.10 n Volume of KMnO 3,收M L Moles of KMnO Multiply the volume of KmnOs solution, in liters, by the CO I of the KMnOs solution. o 0186 mM x 0.0034S L M/L Moles of KMnO Experimental Moles of Fo in sample Since there is a mole ratio of 5 Fe to 1 Mn in the balanced chemical reaction, multiply the number of moles of KMnO by 5 to calculate the number of moles of Fe? in the sample. o. 0003243 Moles of Fo0000 324 moles Mass of Fo in sample Multiply the moles of Fe, in the sample by the atomic mass of Fe、 (The loss of the 2 electrons is negligible compared to the mass of an iron atom, so the mass of an ion is experimentally the same as the mass of the atom.) .0005 945gm Mass of Fe in sample: Experimental percent mass of Fe Divide the mass of Fe?- in the sample by the mass of Fe(NH)z(S04) 6H-0 weighed out on the balance in step 2 of the procedure. Multiply by 100. 0.01309 、 0,IKo Expermental percent mass of Fo'in sample olo 7 79 0.0180x 0.000324 ols 79
Solved
Chemistry
1 Answer
Nataki Medina

Login to view answer.